[SIZE=+4]Supplement Spotlight: Hawthorn[/SIZE]
- Article originally appeared online December 2, 2006
Dana Houser, MD, MHSA, CISSN
[SIZE=-3]Author’s Note: The author has made every effort to ensure the accuracy and completeness of the information presented in this article. In fact, I am willing to suggest this to be the most complete piece offered on this supplement to date. However, the author, nor Anablolic Minds can or should be held responsible for the continued currency of the information, any inadvertent errors or omissions, or the application of this information. Therefore, all of the aforementioned parties shall have NO liability to any person or entity with regard to claims, loss, or damage caused or alleged to be caused, directly or indirectly, by the use of information contained herein.
The inclusion of any product in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of authority to exercise any right or privilege protected by such patent or trademark. All such rights or trademarks are vested in the patent or trademark owner, and no other person may exercise the same without expressed permission, authority, or license secured from such patent or trademark owner.
The inclusion of a brand name does NOT mean the author nor any of the aforementioned parties has any particular knowledge that the brand listed has properties different from other brands of the same product, nor should its inclusion be interpreted as an endorsement by the author or said parties. Similarly, the fact that a particular brand does not indicate the product has been judged to be in any way unsatisfactory or unacceptable. Further, no official support or endorsement of this article by any federal or state agency or pharmaceutical company is intended or inferred.
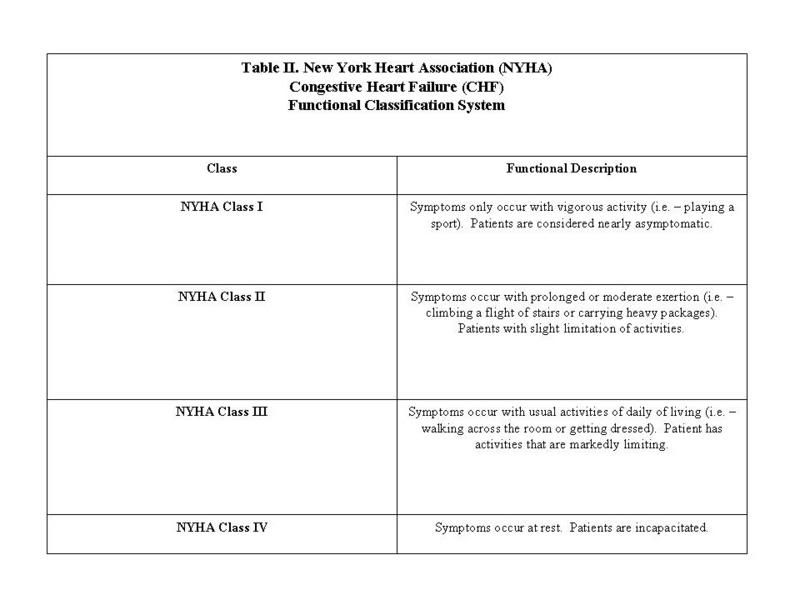
You will read herein about signs, symptoms, and a classification system as applied by the New York Heart Association (NYHA) in regards to congestive heart failure (CHF). This is NOT to replace the diagnostic and treatment modality supplied by an examining physician, but potentially encourages accurate discussion of complimentary and alternative medicine (CAM) treatment strategies as an adjunct to potential care received.[/SIZE]
[SIZE=+2]Introduction[/SIZE]
The hawthorn tree has a storied past that includes significant acknowledgement in Christian tradition. The name Crataegus (genus of
Hawthorn species – see later) is said to be a Greek derivation from the word for “strong” or “always having been there.” It is suggested that the crown of thorns placed on the head of Christ was of its origin. In fact, a grove of hawthorn trees still stands outside of Jerusalem on the Mount of Olives.
Medicinal use of Hawthorn can be traced back to the 1st Century AD. Dioscorides, Pliny, and Galen all referred to hawthorn in their writings but gave little explanation of its use. The use of its leaves and flowers in particular, as a remedy for heart disorders dates back to the nineteenth century. Quercetanus, physician of Henry IV of France, used syrup made from hawthorn fruit to treat heart ailments.
Many species of hawthorn are distributed throughout the moderate zones of the Northern Hemisphere. The fruit, leaves, and flowers of hawthorn species have been used medicinally for centuries and were in use by North American Indians before the arrival of Europeans to treat sleep and digestive disorders, as well as a diuretic for kidney and bladder disorders (later this effect would likely contribute to both its blood pressure lowering properties as well as the heart tonic effect described well before). Hawthorrn has a long history of use in traditional Chinese medicine.
Recently, it has gained acclaim as a potential adjunct in the battle against hypertension (high blood pressure). It contains rutin, magnesium, chromium, catechin, and several other phytochemicals, all of which work to combat high blood pressure.
[SIZE=+2]By Any Other Name[/SIZE]
It is all too often that I hear complaints that these scientific names continue to crop up and I agree, but Hawthorn is rather basic. It tends to go by one of its 3 predominate genus and/or species names. You may see Hawthorn referenced in supplements by any of the following:
- Crataegus monogyna
- Crataegus laevigata
- Crataegus oxyacantha
- Crataegus oxyacanthoides
Please note that the last two on this list of four reference the same species. How these names come about sometime is very funny, these just stem from standard botanical classification schemes as discussed below.
[SIZE=+2]Family, Genus & Species[/SIZE]
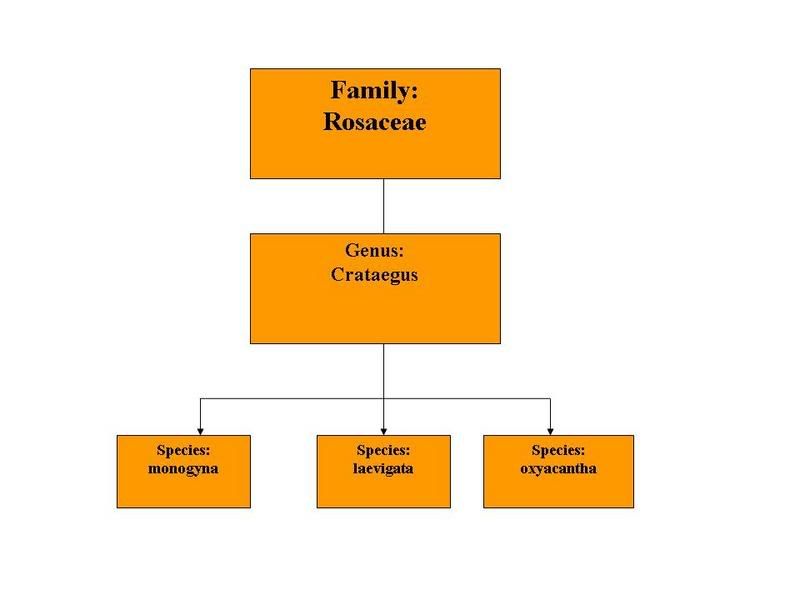
Although Crataegus oxycantha and Crataegus monogyna, species of European shrubs, are generally named as the source of hawthorn extracts, Petrides (1086) points out that the number of hawthorn species native to North America have been variously estimated from 100 to 1000 with considerable hybridization, making identification difficult. It is likely that all species of these shrubs and low trees possess similar chemical constituents. Chang and colleagues (2002) name 6 species of Hawthorn used medicinally around the world. Hawthorn are members of the Rosaceae family.
[SIZE=+2]Plant Part Used[/SIZE]
Hawthorn is a European shrub with thorny branches. Much of its action on the cardiovascular system is attributed to its flavanoid concentration. Its flowers & leaves
[SIZE=+2]Active Constituents[/SIZE]
The best cited active constituents are the
- Flavanoids (1.8%)
Hyperoside (0.28%)
Rutin (0.17%)
- Oligomeric Procyanidins (2-3%)
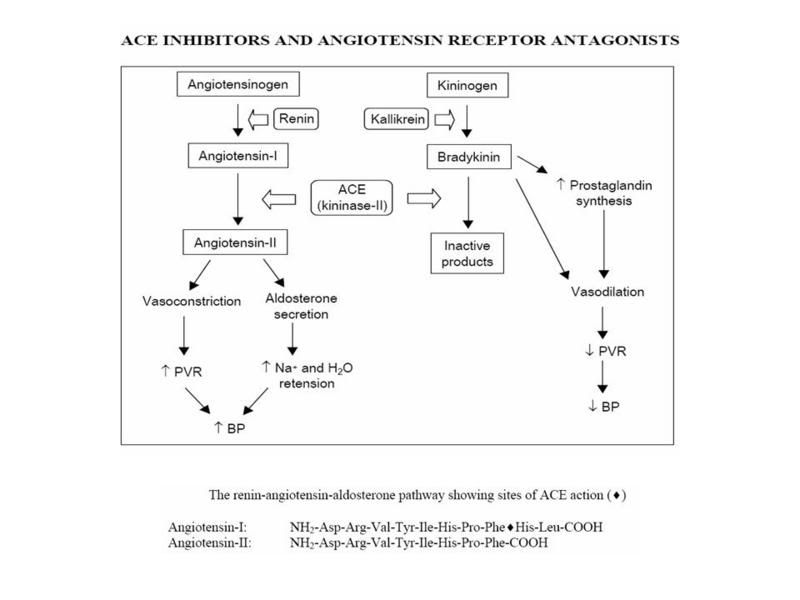
The procyanidins and flavanoids in hawthorn are thought to determine its therapeutic actions. As seen below, these substances are projected to be responsible for vasodilatory effects thought to be responsible for the efficacious benefit seen in cardiac insufficient states (i.e. – CHF, HYHA class I-II).
Other cited active constituents include:
- Catechins
- Triterpenoids
- Aromatic Carboxylic Acids
- Cardioactive Amines
- Amino & Purine Derivatives
- Others
- Article originally appeared online December 2, 2006
Dana Houser, MD, MHSA, CISSN
[SIZE=-3]Author’s Note: The author has made every effort to ensure the accuracy and completeness of the information presented in this article. In fact, I am willing to suggest this to be the most complete piece offered on this supplement to date. However, the author, nor Anablolic Minds can or should be held responsible for the continued currency of the information, any inadvertent errors or omissions, or the application of this information. Therefore, all of the aforementioned parties shall have NO liability to any person or entity with regard to claims, loss, or damage caused or alleged to be caused, directly or indirectly, by the use of information contained herein.
The inclusion of any product in respect to which patent or trademark rights may exist shall not be deemed, and is not intended as, a grant of authority to exercise any right or privilege protected by such patent or trademark. All such rights or trademarks are vested in the patent or trademark owner, and no other person may exercise the same without expressed permission, authority, or license secured from such patent or trademark owner.
The inclusion of a brand name does NOT mean the author nor any of the aforementioned parties has any particular knowledge that the brand listed has properties different from other brands of the same product, nor should its inclusion be interpreted as an endorsement by the author or said parties. Similarly, the fact that a particular brand does not indicate the product has been judged to be in any way unsatisfactory or unacceptable. Further, no official support or endorsement of this article by any federal or state agency or pharmaceutical company is intended or inferred.
You will read herein about signs, symptoms, and a classification system as applied by the New York Heart Association (NYHA) in regards to congestive heart failure (CHF). This is NOT to replace the diagnostic and treatment modality supplied by an examining physician, but potentially encourages accurate discussion of complimentary and alternative medicine (CAM) treatment strategies as an adjunct to potential care received.[/SIZE]
[SIZE=+2]Introduction[/SIZE]
The hawthorn tree has a storied past that includes significant acknowledgement in Christian tradition. The name Crataegus (genus of
Hawthorn species – see later) is said to be a Greek derivation from the word for “strong” or “always having been there.” It is suggested that the crown of thorns placed on the head of Christ was of its origin. In fact, a grove of hawthorn trees still stands outside of Jerusalem on the Mount of Olives.
Medicinal use of Hawthorn can be traced back to the 1st Century AD. Dioscorides, Pliny, and Galen all referred to hawthorn in their writings but gave little explanation of its use. The use of its leaves and flowers in particular, as a remedy for heart disorders dates back to the nineteenth century. Quercetanus, physician of Henry IV of France, used syrup made from hawthorn fruit to treat heart ailments.
Many species of hawthorn are distributed throughout the moderate zones of the Northern Hemisphere. The fruit, leaves, and flowers of hawthorn species have been used medicinally for centuries and were in use by North American Indians before the arrival of Europeans to treat sleep and digestive disorders, as well as a diuretic for kidney and bladder disorders (later this effect would likely contribute to both its blood pressure lowering properties as well as the heart tonic effect described well before). Hawthorrn has a long history of use in traditional Chinese medicine.
Recently, it has gained acclaim as a potential adjunct in the battle against hypertension (high blood pressure). It contains rutin, magnesium, chromium, catechin, and several other phytochemicals, all of which work to combat high blood pressure.
[SIZE=+2]By Any Other Name[/SIZE]
It is all too often that I hear complaints that these scientific names continue to crop up and I agree, but Hawthorn is rather basic. It tends to go by one of its 3 predominate genus and/or species names. You may see Hawthorn referenced in supplements by any of the following:
- Crataegus monogyna
- Crataegus laevigata
- Crataegus oxyacantha
- Crataegus oxyacanthoides
Please note that the last two on this list of four reference the same species. How these names come about sometime is very funny, these just stem from standard botanical classification schemes as discussed below.
[SIZE=+2]Family, Genus & Species[/SIZE]
Although Crataegus oxycantha and Crataegus monogyna, species of European shrubs, are generally named as the source of hawthorn extracts, Petrides (1086) points out that the number of hawthorn species native to North America have been variously estimated from 100 to 1000 with considerable hybridization, making identification difficult. It is likely that all species of these shrubs and low trees possess similar chemical constituents. Chang and colleagues (2002) name 6 species of Hawthorn used medicinally around the world. Hawthorn are members of the Rosaceae family.

[SIZE=+2]Plant Part Used[/SIZE]
Hawthorn is a European shrub with thorny branches. Much of its action on the cardiovascular system is attributed to its flavanoid concentration. Its flowers & leaves
[SIZE=+2]Active Constituents[/SIZE]
The best cited active constituents are the
- Flavanoids (1.8%)
Hyperoside (0.28%)
Rutin (0.17%)
- Oligomeric Procyanidins (2-3%)
The procyanidins and flavanoids in hawthorn are thought to determine its therapeutic actions. As seen below, these substances are projected to be responsible for vasodilatory effects thought to be responsible for the efficacious benefit seen in cardiac insufficient states (i.e. – CHF, HYHA class I-II).
Other cited active constituents include:
- Catechins
- Triterpenoids
- Aromatic Carboxylic Acids
- Cardioactive Amines
- Amino & Purine Derivatives
- Others