I'm putting together a thread here to help you all understand the basics of interpreting blood work. I plan to have a series of these threads, but i want to focus initially on Hepatic/Biliary function. As you all know, these blood work factors are important to monitor while on cycle, or if you have some concurrent medical issue. I'll answer any questions about bloodwork and such that you may have to the best of my abilities. 
Steroids with Michael Scally, MD
Oral Anabolic Steroids, Liver Enzyme Tests and Liver Function
by Michael C. Scally, M.D.Author of eBook Human Experimentation in Anabolic Steroid Research by Michael Scally, M.D.
Harvard Medical School - M.D.; Harvard-M.I.T. Program In Health Science & Technology
Massachusetts Institute of Technology, B.S. Chemistry/LIfe Sciences
Dr. Scally early on recognized the lack of research and treatment for individuals using anabolic-androgenic steroids (AAS). He has remained as the sole physician by reputation and publication to actively pursue and advocate the proper use of AAS to optimize health. Dr. Scally has personally cared for thousands of individuals using AAS. His protocol for Anabolic Steroid Induced Hypogonadism has been presented before the Endocrine Society, American Association of Clinical Endocrinologists, American College of Sports Medicine, & International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV.
Question:
Do oral steroids have long-term effects on liver function long after they have been discontinued? I have done quite a few cycles of anadrol and dianabol in the past. But I haven’t done any oral AAS, prohormones, legal or otherwise in several years and my liver function tests are still elevated (AST and ALT). They are about double the top of the normal range. Can any other factors account for this e.g. dietary supplements, genetics, intense physical exercise, heavy childhood use of NSAIDs?
Response:
Mild elevations in liver chemistry tests such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) can reveal serious underlying conditions or have transient and benign etiologies. There are no controlled clinical trials examining the optimal approach for evaluating serum liver chemistries. The American Gastroenterological Association guideline regarding the evaluation and management of abnormal liver chemistry tests proposes a practical, algorithmic approach when the history and physical examination do not reveal the cause.
The history should be thorough, with special attention given to the use of medications, vitamins, herbs, drugs, and alcohol; family history; and any history of blood-product transfusions.[1] In addition to liver chemistries, an initial serologic evaluation includes a prothrombin time; albumin; complete blood count with platelets; hepatitis A, B, and C serologies; and iron studies. The most common causes of elevated aminotransferase levels include alcohol-related liver injury, chronic hepatitis B and C, autoimmune hepatitis, hepatic steatosis (fatty infiltration of the liver), nonalcoholic steatohepatitis, hemochromatosis, Wilson's disease, alpha1-antitrypsin deficiency, and celiac sprue.
Depending on the etiology, management strategies may include cessation of alcohol use, attention to medications, control of diabetes, and modification of lifestyle factors such as obesity. If elevations persist after an appropriate period of observation, further testing may include ultrasonography, other serum studies, and in some cases, liver biopsy.[2] Isolated alterations of biochemical markers of liver damage in a seemingly healthy patient often represent a challenge even for the experienced clinician and usually set off a battery of further, costly tests and consultations that may ultimately prove unnecessary.
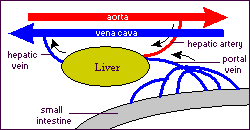
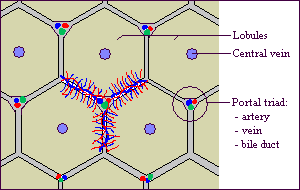
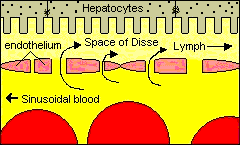
The liver is the largest and most metabolically complex organ in humans. The liver receives a dual blood supply. The portal vein drains the splanchnic, viscera, circulation and provides 75% of the total blood flow. The hepatic artery provides the remaining 25%. The hepatic vein carries all efferent blood to the inferior vena cava. Rich supplies of lymphatic vessels also drain the liver.
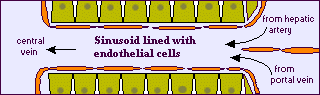
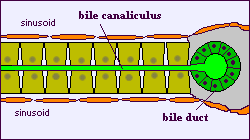
The liver is a complex organ with interdependent metabolic, excretory, and defense functions. Hepatocytes make up the bulk of the organ. Sinusoidal lining cells comprise at least four distinct populations: endothelial cells, Kupffer's cells, perisinusoidal fat-storing cells and pit cells. Endothelial cells are responsible for endocytosis of molecules and particles, and play a role in lipoprotein metabolism. Spindle-shaped Kupffer's cells are tissue macrophages. Perisinusoidal fat-storing cells (Ito cells) store vitamin A. Pit cells are large, granular lymphocytes, which function as natural killer cells.
The liver plays a central role in carbohydrate, protein, and fat metabolism. It stabilizes glucose level by taking up and storing glucose as glycogen (glycogenesis), breaking glycogen down to glucose (glycogenolysis), and forming glucose from noncarbohydrate sources (gluconeogenesis). The liver synthesizes the majority of proteins that circulate in the plasma, including albumin and most of the globulins other than gamma globulins. It is responsible for synthesizing and secreting bile and plasma proteins, including clotting factors. The liver is the site of most amino acid interconversions and catabolism. Amino acid deamination produces urea and esterification of fatty acids produces triglycerides. The liver packages triglycerides with cholesterol, phospholipids, and an apoprotein into a lipoprotein. The lipoprotein enters blood for utilization or storage in adipocytes. Most cholesterol synthesis takes place in the liver.
The liver detoxifies noxious substances arriving from the splanchnic (viscera) circulation, preventing them from entering the systemic circulation. This particularly makes the liver susceptible to drug-induced injury. The liver converts some lipophilic compounds into more water-soluble agents and others to less active agents. In conjunction with the spleen, it is involved in the destruction and reclamation of spent red blood cells.
Prior to a discussion of liver pathology, it is important to have an understanding in the interpretation of laboratory tests. Normal refers to a theoretical frequency distribution for a set of variable data, usually represented by a bell-shaped curve symmetrical about the mean. Laboratory values for a reference range are from a group of healthy individuals with no known factors (medications, illness, genetics, etc.) that would influence the outcome of the testing. The reference range for a particular laboratory test is dependent upon a given subpopulation (e.g., male, female, or children) and the testing laboratory or manufacturer. Federal regulations require laboratories to adhere to certain standards. "Prior to reporting patient test results, the laboratory must verify or establish, for each method, the performance specifications for the following performance characteristics: accuracy; precision; analytical sensitivity and specificity, if applicable; the reportable range of patient test results; the reference range(s) (normal values); and any other applicable performance characteristic."[3] The normal reference range typically refers to the mean or average +2 standard deviations.[4] Interpretation of results is being either within, normal, for a value falling within this bell-shaped curve (reference range) or outside, abnormal, the reference range. Accordingly, 2.5% of normal patients have "abnormal" aminotransferase levels.
A basic tenet, standard practice, of medicine is that interpretation of results is within the framework of a patient's medical condition and treatment, the overall health of the patient.[5] Physicians are taught to think about clinical testing in terms of the clinical significance (particularly, predictive value) of a given test in a given situation. All tests have strengths and limitations for their use in reaching a certain diagnosis or making a causal inference. The risk of a test is seldom inherent in the test itself, but rather is a function of the context in which use of the test is providing information for medical decision-making. Many factors affect test results including sex, medications, overall health of the individual, temporal influences, and variations in laboratory techniques. Thus, in terms of diagnosis, interpretation of a diagnostic test is in the context of history, examination, other tests, and other relevant medical considerations.[6] The proper and correct interpretation for a test is within the situational context.
Levels of serum liver enzymes are indications of hepatocyte integrity or cholestasis rather than liver function. A change in serum protein levels or clotting times may be associated with a decrease in liver functioning mass, although neither is specific for liver disease. No single or simple test assesses overall liver pathology. Use of several screening tests improves the detection of hepatobiliary abnormalities, differentiates the basis for clinically suspected disease, and determines the severity of liver disease (hepatocytes (hepatocellular dysfunction), the biliary excretory apparatus (cholestasis), and the vascular system (portal hypertension)).
The widespread availability and use of serum blood chemistries for screening both symptomatic and asymptomatic patients has resulted in a dramatic increase in the number of normal and abnormal liver chemistry tests requiring interpretation by physicians. A number of review articles on the evaluation of abnormal liver function tests are available on the internet.[7] Aminotransferases (transaminase) include alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Both are exquisitely sensitive indicators of hepatocellular injury and provide the best guide to hepatocellular necrosis/inflammation.[8]
ALT (8-37 IU/L) is present in hepatocytes (liver cells) and is reliable for routine screening for liver disease. It is also called serum glutamate pyruvate transaminase (SGPT) or alanine aminotransferase (ALAT). When a cell is damaged, it leaks this enzyme into the blood, where it is measured. ALT rises dramatically in acute liver damage, such as viral hepatitis or paracetamol (acetaminophen) overdose. The highest level of ALT is in the liver, and levels of this enzyme are accordingly more specific indicators of liver injury. The magnitude of the elevation has no prognostic value and does not correlate with the degree of liver damage.
AST (10-34 IU/L), also called serum glutamic oxaloacetic transaminase (SGOT) or aspartate aminotransferase (ASAT/AAT) is similar to alanine transaminase (ALT) in that it is another enzyme associated with liver parenchymal cells. AST is present, in decreasing order of concentration, in the liver, cardiac muscle, skeletal muscle, kidneys, brain, pancreas, lungs, leukocytes, and erythrocytes. AST levels thus rise in MI, heart failure, muscle injury, CNS disease, and other nonhepatic disorders. AST is relatively nonspecific, but high levels indicate liver cell injury. In most liver diseases, the AST increase is less than that of ALT (AST/ALT ratio < 1).
Both aminotransferases are normally present in serum at low levels, usually less than 30 to 40 IU/L. The normal range varies widely among laboratories. The following table lists factors affecting AST and ALT serum activity, other than liver injury.[9] Release of both enzymes into the blood occurs in increasing amounts with liver cell membrane damage. Necrosis of liver cells is not required for the release of the aminotransferases. In fact, there is poor correlation between the degree of liver-cell damage and the level of the aminotransferases. The magnitude of elevation covers a very wide range. Levels <100 IU are common and nonspecific, and often have no clinical significance; levels of 100-300 IU are seen in numerous mild/moderate inflammatory processes. In acute viral or drug hepatitis aminotransferase levels are typically in the 500-1,500 IU range, but in alcoholic hepatitis they are usually <300 IU, even if the disease is severe. Values >3,000 IU usually are seen only in acute toxic necrosis or severe hypoxia ("shock liver," "ischemic hepatitis"); in both disorders levels typically plummet within two to three days, whereas values fall more slowly in viral hepatitis. Aminotransferase levels are variable in biliary obstruction but usually remain <200 IU, except with acute passage of common duct stone, characterized by a sudden rise to hepatitic levels and a rapid fall over the next one to two days.
Factor: Time of day
AST: 45% variation during day; highest in afternoon, lowest at night
ALT: No significant difference between 0900 and 2100;
Comment: similar in liver disease and health
Factor: Day-to-day
AST: 5–10% variation from one day to next
ALT: 10–30% variation from one day to next
Comment: Similar in liver disease and health, and in elderly and young
Factor: Race/gender
AST: 15% higher in African-American men
ALT: No significant difference between African-American, other women
Factor: BMI (body mass index)
AST: 40–50% higher with high BMI
ALT: 40–50% higher with high BMI
Comment: Direct relationship between weight and AST, ALT
Factor: Meals
AST: No effect
ALT: No effect
Factor: Exercise
AST: Threefold increase with strenuous exercise
20% lower in those who exercise at usual levels than in those who do not exercise or exercise more strenuously than usual
ALT: Effect of exercise seen predominantly in men; minimal difference in women (<10%). Enzymes increase more with strength training
Factor: Specimen storage
AST: Stable at room temp for 3 days, in refrigerator for 3 weeks (<10% decrease); stable for years frozen (10–15% decrease)
ALT: Stable at room temperature for 3 days, in refrigerator for 3 weeks (10–15% decrease); marked decrease with freezing/thawing
Comment: Stability based on serum separated from cells; stable for 24 h in whole blood, marked increase after 24 h
Factor: Hemolysis, hemolytic anemia:
AST: Significant increase
ALT: Moderate increase attributable to release from red cell
Comment: Dependent on degree of hemolysis; usually several fold lower than increases in lactate dehydrogenase (LDH)
Factor: Muscle injury:
AST: Significant increase
ALT: Moderate increase
Comment: Related to amount of increase in creatine kinase (CK)
Other biochemical tests of interest are γ-glutamyl transpeptidase (GGT), lactic dehydrogenase (LDH), alkaline phosphatase (ALP), albumin, and bilirubin. Corresponding changes in the serum levels of these markers assist in defining the etiology. γ-Glutamyl transpeptidase (GGT), also known as γ-glutamyltransferase, is present in the liver, pancreas, and kidney. GGT transfers the γ-glutamyl group from one peptide to another or to an L-amino acid. GGT levels (0-51 IU/L) are elevated in diseases of the liver, biliary tract, and pancreas with obstruction of the common bile duct. Drug use and alcohol (acute and chronic) ingestion also elevate GGT. GGT may be elevated with even minor, sub-clinical levels of liver dysfunction. Alkaline phosphatase (ALP) is an enzyme in the cells lining the biliary ducts of the liver. ALP levels (44-147 IU/L) in plasma will rise with large bile duct obstruction, intrahepatic cholestasis, or infiltrative diseases of the liver. ALP is also present in bone. Serum γ-glutamyl transpeptidase (GGT) activity correlates closely with the activities of alkaline phosphatase (ALP) in various forms of liver disease. Maximum elevations of the enzyme activities are observed in diseases that affect the biliary tract. Compared with ALP, GGT is generally increased to a greater extent and is thus the most sensitive indicator of biliary-tract disease.
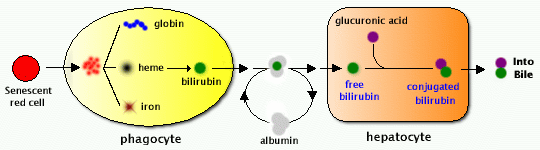
Lactic dehydrogenase (LDH) is commonly included in routine analysis, is insensitive as an indicator of hepatocellular injury, but is better as a marker for hemolysis, myocardial infarction (heart attack), or pulmonary embolism. LDH can be quite high with malignancies involving the liver. Albumin (3.9-5.0 g/dL) is a protein made specifically by the liver, and can be measured cheaply and easily. It is the main constituent of total protein; the remaining fraction is called globulin (including the immunoglobulins). Bilirubin is a breakdown product of heme (a part of hemoglobin in red blood cells). The liver is responsible for clearing the blood of bilirubin. Bilirubin is taken up into hepatocytes, conjugated (modified to make it water-soluble), and secreted into the bile, which is excreted into the intestine. Increased total bilirubin causes jaundice, and can signal a number of problems.
Elevated serum aminotransferase levels, especially aspartate aminotransferase levels, may be caused by disorders that affect organs or tissues other than the liver, with the most common being striated muscle. Conditions or activities that can cause such elevations include subclinical inborn errors of muscle metabolism; acquired muscle disorders, such as polymyositis; and exercise. If striated muscle is the source of increased aminotransferase levels, serum levels of creatine kinase will be elevated to the same degree or to an even higher degree.
Creatine kinase (CK), also known as phosphocreatine kinase or creatine phosphokinase (CPK) is an enzyme that catalyses the conversion of creatine to phosphocreatine. In tissues that consume ATP rapidly, especially skeletal muscle, but also brain and smooth muscle, phosphocreatine serves as an energy reservoir for the rapid regeneration of ATP. Clinically, creatine kinase is assayed in blood tests as a marker of myocardial infarction (heart attack), rhabdomyolysis (muscle breakdown), and in acute renal failure. Numerous studies have evaluated changes in CK activity after exercise and found that it differs markedly according to exercise conditions. In isometric muscle contraction exercise, peak serum CK activity is observed relatively early, 24-48 hours after exercise, whereas it is seen 3-7 days after exercise in eccentric muscle contraction exercise, and a biphasic pattern is observed in weight training.
Toxic effects of AAS on the liver are primarily due to 17α-alkylated steroids and reported to include increased enzyme activities, cholestasis, peliosis hepatis adenoma, and even case reports of carcinoma.[10] The use of anabolic steroids is common among athletes, particularly bodybuilders. Prior reports of anabolic steroid-induced hepatotoxicity based on elevated aminotransferase levels have been overstated. Such reports may have misled the medical community to emphasize steroid-induced hepatotoxicity when interpreting elevated aminotransferase levels and disregard muscle damage. Levels of both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) may increase with strenuous exercise. Evaluating enzyme elevations in patients who use anabolic steroids, physicians should consider the CK and GGT levels as essential elements in distinguishing muscle damage from liver damage
Steroids with Michael Scally, MD
Oral Anabolic Steroids, Liver Enzyme Tests and Liver Function
by Michael C. Scally, M.D.Author of eBook Human Experimentation in Anabolic Steroid Research by Michael Scally, M.D.
Harvard Medical School - M.D.; Harvard-M.I.T. Program In Health Science & Technology
Massachusetts Institute of Technology, B.S. Chemistry/LIfe Sciences
Dr. Scally early on recognized the lack of research and treatment for individuals using anabolic-androgenic steroids (AAS). He has remained as the sole physician by reputation and publication to actively pursue and advocate the proper use of AAS to optimize health. Dr. Scally has personally cared for thousands of individuals using AAS. His protocol for Anabolic Steroid Induced Hypogonadism has been presented before the Endocrine Society, American Association of Clinical Endocrinologists, American College of Sports Medicine, & International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV.
Question:
Do oral steroids have long-term effects on liver function long after they have been discontinued? I have done quite a few cycles of anadrol and dianabol in the past. But I haven’t done any oral AAS, prohormones, legal or otherwise in several years and my liver function tests are still elevated (AST and ALT). They are about double the top of the normal range. Can any other factors account for this e.g. dietary supplements, genetics, intense physical exercise, heavy childhood use of NSAIDs?
Response:
Mild elevations in liver chemistry tests such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) can reveal serious underlying conditions or have transient and benign etiologies. There are no controlled clinical trials examining the optimal approach for evaluating serum liver chemistries. The American Gastroenterological Association guideline regarding the evaluation and management of abnormal liver chemistry tests proposes a practical, algorithmic approach when the history and physical examination do not reveal the cause.
The history should be thorough, with special attention given to the use of medications, vitamins, herbs, drugs, and alcohol; family history; and any history of blood-product transfusions.[1] In addition to liver chemistries, an initial serologic evaluation includes a prothrombin time; albumin; complete blood count with platelets; hepatitis A, B, and C serologies; and iron studies. The most common causes of elevated aminotransferase levels include alcohol-related liver injury, chronic hepatitis B and C, autoimmune hepatitis, hepatic steatosis (fatty infiltration of the liver), nonalcoholic steatohepatitis, hemochromatosis, Wilson's disease, alpha1-antitrypsin deficiency, and celiac sprue.
Depending on the etiology, management strategies may include cessation of alcohol use, attention to medications, control of diabetes, and modification of lifestyle factors such as obesity. If elevations persist after an appropriate period of observation, further testing may include ultrasonography, other serum studies, and in some cases, liver biopsy.[2] Isolated alterations of biochemical markers of liver damage in a seemingly healthy patient often represent a challenge even for the experienced clinician and usually set off a battery of further, costly tests and consultations that may ultimately prove unnecessary.
The liver is the largest and most metabolically complex organ in humans. The liver receives a dual blood supply. The portal vein drains the splanchnic, viscera, circulation and provides 75% of the total blood flow. The hepatic artery provides the remaining 25%. The hepatic vein carries all efferent blood to the inferior vena cava. Rich supplies of lymphatic vessels also drain the liver.
The liver is a complex organ with interdependent metabolic, excretory, and defense functions. Hepatocytes make up the bulk of the organ. Sinusoidal lining cells comprise at least four distinct populations: endothelial cells, Kupffer's cells, perisinusoidal fat-storing cells and pit cells. Endothelial cells are responsible for endocytosis of molecules and particles, and play a role in lipoprotein metabolism. Spindle-shaped Kupffer's cells are tissue macrophages. Perisinusoidal fat-storing cells (Ito cells) store vitamin A. Pit cells are large, granular lymphocytes, which function as natural killer cells.
The liver plays a central role in carbohydrate, protein, and fat metabolism. It stabilizes glucose level by taking up and storing glucose as glycogen (glycogenesis), breaking glycogen down to glucose (glycogenolysis), and forming glucose from noncarbohydrate sources (gluconeogenesis). The liver synthesizes the majority of proteins that circulate in the plasma, including albumin and most of the globulins other than gamma globulins. It is responsible for synthesizing and secreting bile and plasma proteins, including clotting factors. The liver is the site of most amino acid interconversions and catabolism. Amino acid deamination produces urea and esterification of fatty acids produces triglycerides. The liver packages triglycerides with cholesterol, phospholipids, and an apoprotein into a lipoprotein. The lipoprotein enters blood for utilization or storage in adipocytes. Most cholesterol synthesis takes place in the liver.
The liver detoxifies noxious substances arriving from the splanchnic (viscera) circulation, preventing them from entering the systemic circulation. This particularly makes the liver susceptible to drug-induced injury. The liver converts some lipophilic compounds into more water-soluble agents and others to less active agents. In conjunction with the spleen, it is involved in the destruction and reclamation of spent red blood cells.
Prior to a discussion of liver pathology, it is important to have an understanding in the interpretation of laboratory tests. Normal refers to a theoretical frequency distribution for a set of variable data, usually represented by a bell-shaped curve symmetrical about the mean. Laboratory values for a reference range are from a group of healthy individuals with no known factors (medications, illness, genetics, etc.) that would influence the outcome of the testing. The reference range for a particular laboratory test is dependent upon a given subpopulation (e.g., male, female, or children) and the testing laboratory or manufacturer. Federal regulations require laboratories to adhere to certain standards. "Prior to reporting patient test results, the laboratory must verify or establish, for each method, the performance specifications for the following performance characteristics: accuracy; precision; analytical sensitivity and specificity, if applicable; the reportable range of patient test results; the reference range(s) (normal values); and any other applicable performance characteristic."[3] The normal reference range typically refers to the mean or average +2 standard deviations.[4] Interpretation of results is being either within, normal, for a value falling within this bell-shaped curve (reference range) or outside, abnormal, the reference range. Accordingly, 2.5% of normal patients have "abnormal" aminotransferase levels.
A basic tenet, standard practice, of medicine is that interpretation of results is within the framework of a patient's medical condition and treatment, the overall health of the patient.[5] Physicians are taught to think about clinical testing in terms of the clinical significance (particularly, predictive value) of a given test in a given situation. All tests have strengths and limitations for their use in reaching a certain diagnosis or making a causal inference. The risk of a test is seldom inherent in the test itself, but rather is a function of the context in which use of the test is providing information for medical decision-making. Many factors affect test results including sex, medications, overall health of the individual, temporal influences, and variations in laboratory techniques. Thus, in terms of diagnosis, interpretation of a diagnostic test is in the context of history, examination, other tests, and other relevant medical considerations.[6] The proper and correct interpretation for a test is within the situational context.
Levels of serum liver enzymes are indications of hepatocyte integrity or cholestasis rather than liver function. A change in serum protein levels or clotting times may be associated with a decrease in liver functioning mass, although neither is specific for liver disease. No single or simple test assesses overall liver pathology. Use of several screening tests improves the detection of hepatobiliary abnormalities, differentiates the basis for clinically suspected disease, and determines the severity of liver disease (hepatocytes (hepatocellular dysfunction), the biliary excretory apparatus (cholestasis), and the vascular system (portal hypertension)).
The widespread availability and use of serum blood chemistries for screening both symptomatic and asymptomatic patients has resulted in a dramatic increase in the number of normal and abnormal liver chemistry tests requiring interpretation by physicians. A number of review articles on the evaluation of abnormal liver function tests are available on the internet.[7] Aminotransferases (transaminase) include alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Both are exquisitely sensitive indicators of hepatocellular injury and provide the best guide to hepatocellular necrosis/inflammation.[8]
ALT (8-37 IU/L) is present in hepatocytes (liver cells) and is reliable for routine screening for liver disease. It is also called serum glutamate pyruvate transaminase (SGPT) or alanine aminotransferase (ALAT). When a cell is damaged, it leaks this enzyme into the blood, where it is measured. ALT rises dramatically in acute liver damage, such as viral hepatitis or paracetamol (acetaminophen) overdose. The highest level of ALT is in the liver, and levels of this enzyme are accordingly more specific indicators of liver injury. The magnitude of the elevation has no prognostic value and does not correlate with the degree of liver damage.
AST (10-34 IU/L), also called serum glutamic oxaloacetic transaminase (SGOT) or aspartate aminotransferase (ASAT/AAT) is similar to alanine transaminase (ALT) in that it is another enzyme associated with liver parenchymal cells. AST is present, in decreasing order of concentration, in the liver, cardiac muscle, skeletal muscle, kidneys, brain, pancreas, lungs, leukocytes, and erythrocytes. AST levels thus rise in MI, heart failure, muscle injury, CNS disease, and other nonhepatic disorders. AST is relatively nonspecific, but high levels indicate liver cell injury. In most liver diseases, the AST increase is less than that of ALT (AST/ALT ratio < 1).
Both aminotransferases are normally present in serum at low levels, usually less than 30 to 40 IU/L. The normal range varies widely among laboratories. The following table lists factors affecting AST and ALT serum activity, other than liver injury.[9] Release of both enzymes into the blood occurs in increasing amounts with liver cell membrane damage. Necrosis of liver cells is not required for the release of the aminotransferases. In fact, there is poor correlation between the degree of liver-cell damage and the level of the aminotransferases. The magnitude of elevation covers a very wide range. Levels <100 IU are common and nonspecific, and often have no clinical significance; levels of 100-300 IU are seen in numerous mild/moderate inflammatory processes. In acute viral or drug hepatitis aminotransferase levels are typically in the 500-1,500 IU range, but in alcoholic hepatitis they are usually <300 IU, even if the disease is severe. Values >3,000 IU usually are seen only in acute toxic necrosis or severe hypoxia ("shock liver," "ischemic hepatitis"); in both disorders levels typically plummet within two to three days, whereas values fall more slowly in viral hepatitis. Aminotransferase levels are variable in biliary obstruction but usually remain <200 IU, except with acute passage of common duct stone, characterized by a sudden rise to hepatitic levels and a rapid fall over the next one to two days.
Factor
AST
ALT
Comments
AST
ALT
Comments
Factor: Time of day
AST: 45% variation during day; highest in afternoon, lowest at night
ALT: No significant difference between 0900 and 2100;
Comment: similar in liver disease and health
Factor: Day-to-day
AST: 5–10% variation from one day to next
ALT: 10–30% variation from one day to next
Comment: Similar in liver disease and health, and in elderly and young
Factor: Race/gender
AST: 15% higher in African-American men
ALT: No significant difference between African-American, other women
Factor: BMI (body mass index)
AST: 40–50% higher with high BMI
ALT: 40–50% higher with high BMI
Comment: Direct relationship between weight and AST, ALT
Factor: Meals
AST: No effect
ALT: No effect
Factor: Exercise
AST: Threefold increase with strenuous exercise
20% lower in those who exercise at usual levels than in those who do not exercise or exercise more strenuously than usual
ALT: Effect of exercise seen predominantly in men; minimal difference in women (<10%). Enzymes increase more with strength training
Factor: Specimen storage
AST: Stable at room temp for 3 days, in refrigerator for 3 weeks (<10% decrease); stable for years frozen (10–15% decrease)
ALT: Stable at room temperature for 3 days, in refrigerator for 3 weeks (10–15% decrease); marked decrease with freezing/thawing
Comment: Stability based on serum separated from cells; stable for 24 h in whole blood, marked increase after 24 h
Factor: Hemolysis, hemolytic anemia:
AST: Significant increase
ALT: Moderate increase attributable to release from red cell
Comment: Dependent on degree of hemolysis; usually several fold lower than increases in lactate dehydrogenase (LDH)
Factor: Muscle injury:
AST: Significant increase
ALT: Moderate increase
Comment: Related to amount of increase in creatine kinase (CK)
Other biochemical tests of interest are γ-glutamyl transpeptidase (GGT), lactic dehydrogenase (LDH), alkaline phosphatase (ALP), albumin, and bilirubin. Corresponding changes in the serum levels of these markers assist in defining the etiology. γ-Glutamyl transpeptidase (GGT), also known as γ-glutamyltransferase, is present in the liver, pancreas, and kidney. GGT transfers the γ-glutamyl group from one peptide to another or to an L-amino acid. GGT levels (0-51 IU/L) are elevated in diseases of the liver, biliary tract, and pancreas with obstruction of the common bile duct. Drug use and alcohol (acute and chronic) ingestion also elevate GGT. GGT may be elevated with even minor, sub-clinical levels of liver dysfunction. Alkaline phosphatase (ALP) is an enzyme in the cells lining the biliary ducts of the liver. ALP levels (44-147 IU/L) in plasma will rise with large bile duct obstruction, intrahepatic cholestasis, or infiltrative diseases of the liver. ALP is also present in bone. Serum γ-glutamyl transpeptidase (GGT) activity correlates closely with the activities of alkaline phosphatase (ALP) in various forms of liver disease. Maximum elevations of the enzyme activities are observed in diseases that affect the biliary tract. Compared with ALP, GGT is generally increased to a greater extent and is thus the most sensitive indicator of biliary-tract disease.
Lactic dehydrogenase (LDH) is commonly included in routine analysis, is insensitive as an indicator of hepatocellular injury, but is better as a marker for hemolysis, myocardial infarction (heart attack), or pulmonary embolism. LDH can be quite high with malignancies involving the liver. Albumin (3.9-5.0 g/dL) is a protein made specifically by the liver, and can be measured cheaply and easily. It is the main constituent of total protein; the remaining fraction is called globulin (including the immunoglobulins). Bilirubin is a breakdown product of heme (a part of hemoglobin in red blood cells). The liver is responsible for clearing the blood of bilirubin. Bilirubin is taken up into hepatocytes, conjugated (modified to make it water-soluble), and secreted into the bile, which is excreted into the intestine. Increased total bilirubin causes jaundice, and can signal a number of problems.
Elevated serum aminotransferase levels, especially aspartate aminotransferase levels, may be caused by disorders that affect organs or tissues other than the liver, with the most common being striated muscle. Conditions or activities that can cause such elevations include subclinical inborn errors of muscle metabolism; acquired muscle disorders, such as polymyositis; and exercise. If striated muscle is the source of increased aminotransferase levels, serum levels of creatine kinase will be elevated to the same degree or to an even higher degree.
Creatine kinase (CK), also known as phosphocreatine kinase or creatine phosphokinase (CPK) is an enzyme that catalyses the conversion of creatine to phosphocreatine. In tissues that consume ATP rapidly, especially skeletal muscle, but also brain and smooth muscle, phosphocreatine serves as an energy reservoir for the rapid regeneration of ATP. Clinically, creatine kinase is assayed in blood tests as a marker of myocardial infarction (heart attack), rhabdomyolysis (muscle breakdown), and in acute renal failure. Numerous studies have evaluated changes in CK activity after exercise and found that it differs markedly according to exercise conditions. In isometric muscle contraction exercise, peak serum CK activity is observed relatively early, 24-48 hours after exercise, whereas it is seen 3-7 days after exercise in eccentric muscle contraction exercise, and a biphasic pattern is observed in weight training.
Toxic effects of AAS on the liver are primarily due to 17α-alkylated steroids and reported to include increased enzyme activities, cholestasis, peliosis hepatis adenoma, and even case reports of carcinoma.[10] The use of anabolic steroids is common among athletes, particularly bodybuilders. Prior reports of anabolic steroid-induced hepatotoxicity based on elevated aminotransferase levels have been overstated. Such reports may have misled the medical community to emphasize steroid-induced hepatotoxicity when interpreting elevated aminotransferase levels and disregard muscle damage. Levels of both aspartate aminotransferase (AST) and alanine aminotransferase (ALT) may increase with strenuous exercise. Evaluating enzyme elevations in patients who use anabolic steroids, physicians should consider the CK and GGT levels as essential elements in distinguishing muscle damage from liver damage