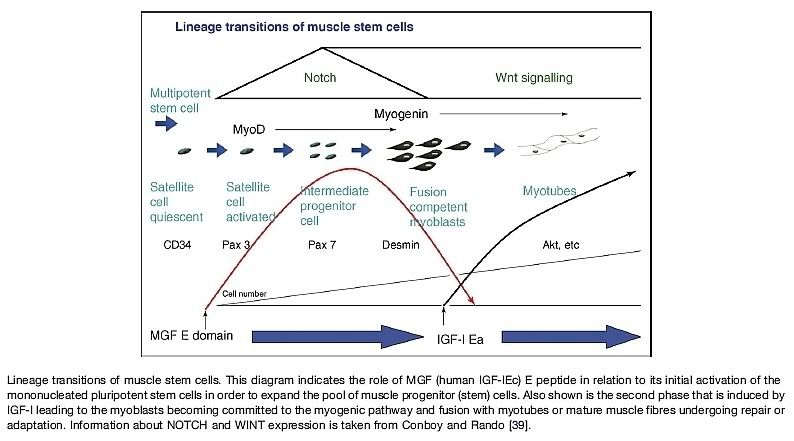
IGF1 is thought to induce muscle hypertrophy, by distinct mechanisms. The IGF1 receptor is a tyrosine-kinase receptor which induces cellular signal transduction chains by adding phosphate groups or “phosphorylating” specific proteins within the cell. Activation of the PI3K/AKT
kinases cause phosphorylation of the FOXO transcription factors, which prevents them from entering the nucleus and promoting the expression of atrophic factors, like MuRF1. The AKT pathway (often called “PKB” instead of “AKT”) also inhibits the secretion of myostatin, thereby increasing both muscle cell differentiation, and protein synthesis.(
ref) Myostatin inhibition results in a positive feedback cycle, since myostatin also inhibits the AKT pathway.(
ref, ref) IGF1 also activates the mTOR pathway, which is well-known to play a central role in muscle growth. Apparently, PI3K activates mTOR by moving tuberous sclerosis complexes (mTOR inhibitors) from the membrane to the cytosol.(
ref) (Independent of growth factors, amino acid availability, especially leucine, regulates mTOR activity,
ref.) For a more detailed discussion of the AKT pathway, see:
Akt: a nexus of growth factor and cytokine signaling in determining muscle mass
For an overview of transcriptional regulation of muscle growth/atrophy pathways,see:
Anabolic and catabolic pathways regulating skeletal muscle mass See, also:
Regulation of Muscle Growth
Until recently the exact roles of these pathways and their relationships to IGF1, the effects of resistance exercise (mechanical load/stretch), and developmental stage have remained mysterious. However, current research involving transgenic models is quickly unraveling these mysteries.
Mechanical Stimuli Activate mTOR Independent of IGF1.
It was observed that mechanical stimulation induced local expression of IGF1 and other growth factors.(
ref) These were accompanied by an increase in kinase phosphorylation signaling, and muscle growth. It was logical to conclude that IGF1 activated the pathways responsible for muscle growth. Subsequent research has cast serious doubts on this conventional theory. In 2004, it was shown that mechanical stimulation activate mTOR growth pathways, completely independent of IGF1 and the PI3K/AKT pathway. Pharmacologically inhibiting PI3K did not alter activation of mTOR. These results were confirmed with AKT-knockout mice (which lack the AKT gene).
Mechanical stimuli regulate rapamycin-sensitive signalling by a phosphoinositide 3-kinase-, protein kinase B- and growth factor-independent mechanism.
“These surprising results indicate that mechanical stimuli are different from insulin-like growth factors in that mTOR-dependent signalling events are regulated via a PI3K/Akt1-independent mechanism. Furthermore, these results indicate that if mechanical stimuli regulate protein synthesis by the release of locally acting factors, then these factors must activate mTOR through a PI3K/ Akt1-independent mechanism. However, in both the co-incubation and conditioned-media experiments, the release of locally acting factors was not sufficient for the activation of mTOR-dependent signalling events, thus suggesting that mechanotransduction (e.g. mechanoreceptor) rather than ligand binding of autocrine/paracrine growth factors as the cause for the induction of the mTOR-dependent signalling events.”
These results were confirmed by a 2009 study,
The role of PI3K in the regulation of mTOR following eccentric contractions:
“In summary, the results from this study indicate that resistance exercise contractions, such as ECs (eccentric contractions), activate mTOR through a PI3K–AKT-independent mechanism.”
In 2007, another transgenic study using mice with a negative IGF1 receptor (one that binds IGF1, but doesn't transduce signals) showed that the hypertrophic effects of mechanical load were NOT mediated by IGF1.(
ref) “We demonstrate that IGF-I receptor-mediated signalling is not necessary for the induction of skeletal muscle hypertrophy in adult mice following a chronic increase in mechanical loading.”
This study has previously been discussed by Dat:
IGF-1 & receptor aren't even needed post-workout
The results of these studies have been further confirmed by a new transgenic study published last year. Researchers conclude, “Acute resistance exercise did not increase either IGF-1 receptor phosphorylation. . . [Furthermore] these data suggest that physiological loading does not lead to the enhanced activation of the PI3K/Akt/mTORC1 axis and that PI3K activation levels play no significant role in adult skeletal muscle growth.”(
ref)
mTOR Causes Muscle Hypertrophy, Not IGF1
Additional studies have confirmed that mTOR plays a central role in muscle growth; but they also confirm that this happens independent of the PI3K/AKT pathway.
A PI3K-independent Activation of mTOR Signaling Is Sufficient to Induce Skeletal Muscle Hypertrophy “In this study, we demonstrate that the overexpression of Rheb induces mTOR signaling through a PI3K/PKB-independent mechanism and that this event is sufficient to induce a robust and cell autonomous hypertrophic response. Furthermore, it was determined that the hypertrophic effects of Rheb occurred through a rapamycin-sensitive mechanism, that mTOR was the rapamycin-sensitive element in skeletal muscle that conferred the hypertrophic response, and that the kinase activity of mTOR was necessary for this event. Combined, these results strongly indicate that a PI3K/PKB-independent activation of mTOR signaling, in skeletal muscle, is sufficient to induce hypertrophy.” The researchers conclude by suggesting that muscle hypertrophy could be induced by the use of mTOR agonists.
What purpose, then, does IGF1 serve?
Obviously it serves many purposes. I would not presume to definitively answer this question. However, it does appear clear from experimental data that the proliferative role of IGF1 is limited to developmental growth and to regenerative repair. IGF1 is necessary for proper development and repair following injury. Young, developing mammals not only need IGF1 for proper development, but overexpression leads to increased growth. The same does not happen in adults overexpressing IGF1. From a transgenic study published in 2010: “In conclusion, these data show that adult non-growing skeletal muscles are refractory to hypertrophy in response to the elevated IGF-1. By contrast, growing muscles respond by activating signalling downstream from the IGF-1 receptor (demonstrated by phosphorylation of Akt, p70
[SUP]S6K[/SUP]) to increase protein accretion by the myofibres. Thus, the IGF-1-mediated hypertrophy evident in adult transgenic muscles results from enhanced increase in muscle mass mainly during the postnatal growth phase.” (
ref)
Am I wasting my time and money on IGF1?
Yes. Anecdotes are not scientific evidence, no matter how loudly they are proclaimed. The previously accepted theory on the role of IGF1 in muscle hypertrophy has been reversed. Many are apparently slow to get the message. This should not come as a surprise to readers of this forum. I merely wanted to give a concise review of some of the recent, relevant literature. All currently available
scientific evidence based on
in vivo studies indicates that IGF1 plays no role in normal, exercise-induced muscle hypertrophy.